The global experiment project at the Royal Society of Chemistry have been getting bigger and better each year.
Thanks in the main to all of you for your participation.
As a result we will now be committing to a new experiment each year in time for British Science Week. All older global experiments will remain open so teachers can use these with new year groups. Eventually we will build up a large suite of mass participation experiment across a range of topics.
As we launch our new expeirment:
Water - a global experiment with hydrogels. I wanted to give Prof. David Evans (RSC Beijing local section and Chemistry Teacher) the final word on how he used the global experiment in Beijing to inspire you all.
Crystallising the links between parents and children in Beijing
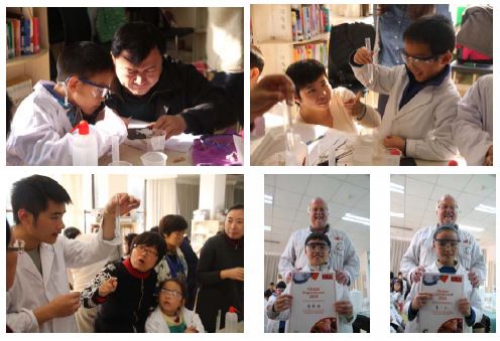
The pressures and distractions of modern life can mean that parents—whether catching up on work emails or updating their social networking status—and their computer-game-playing children do fewer things together than used to be the case. Recently RSC Beijing Local Section joined forces with the Family Education Department of the China National Children’s Center in an effort to reverse this trend, by running Sunday morning practical chemistry classes for young children and their parents working together as a team. Naturally one of the experiments they carried out was
the art of crystallisation - a global experiment. In the first session, each parent–child pair chose one of the samples (table salt, sugar, Epsom salts, potassium nitrate or alum) and measured the average mass to saturate 40 cm3 of tap water. When they compared their results with the average values for the UK given on the global experiment website, they found the values were much lower in each case—indicating just how hard Beijing tap water is (although the relatively low room temperature will also have contributed).

Then they set up their saturated solution and—with a great sense of anticipation–left it to crystallise until the next class. Since this was two weeks away, quite a few couldn’t bear the suspense and repeated the experiment with one of the other solids when they got home so that they could watch developments first hand. The next class started in great excitement as the students and their parents looked at their crop of crystals and compared them with those of other families. On the official global experiment scale of crystal size (from 8–28), the sizes of the biggest crystals were: table salt (23), sugar (25), Epsom salts (28), potassium nitrate (27) and alum (25)—a creditable all-round performance, despite the hard water!
It has been great seeing all the images in Pinterest and following the data as more people take part. The results from over 30,000 participants worldwide is just amazing.
I am looking forward to see some of the same schools and some new ones taking part in our
new experiment - check it out!
Kind regards
Lee Page (Learn Chemistry, Executive)


